A dry cell battery was invented in Japan during the Meiji Era in 1887, In contrast to wet-cell batteries, a dry cell battery uses a paste electrolyte, with only enough moisture to allow current to flow, it can operate in any orientation without spilling, as it contains no free liquid, so it is suitable for portable equipment.
In today’s power savvy world, dry cell is one of many types of electrochemical cells available for consumer use, but it was a great innovation when it was invented.
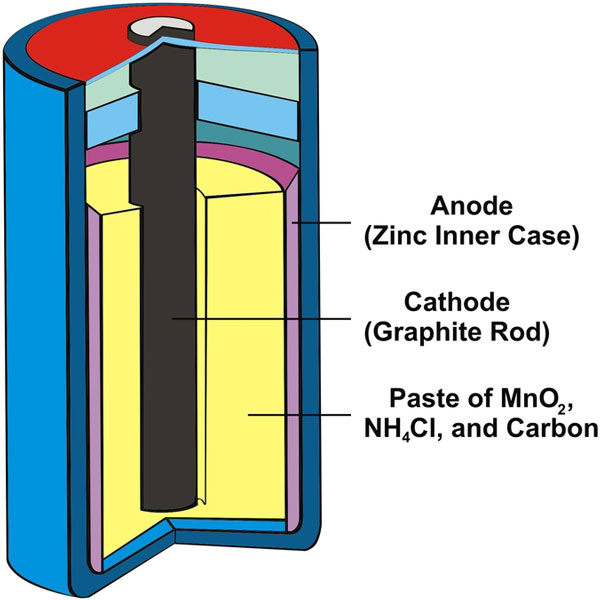

A common dry-cell battery is the zinc-carbon battery, which is an adaptation of a wet cell that is called the Leclanché cell. As you can see from the above illustration, the cell is made up of a zinc compartment acting as the anode. The cathode is a carbon bar, fully surrounded by a paste of carbon, ammonium chloride (NH4Cl) and manganese oxide (MnO2).
Battery manufacturers classify battery types as either primary (single-use disposables) or secondary (rechargeables). Dry cells can be either primary or secondary cells.
Smaller dry-cell batteries, such as alkaline or lithium ion, are typically used in portable electronics, such as toys, phones and laptops
Dry Cell Advantages
When dry cell batteries were first created, they boasted many advantages over wet cell batteries. The first wet cell batteries were often very delicate and could leak from their caustic electrolytes when inverted or simply when moved too vigorously. Dry cell batteries were much less volatile and could survive much harsher treatment. In contemporary times gel batteries have solved most of the worst problems with wet cell batteries, but dry cell batteries still do have advantages in certain applications.
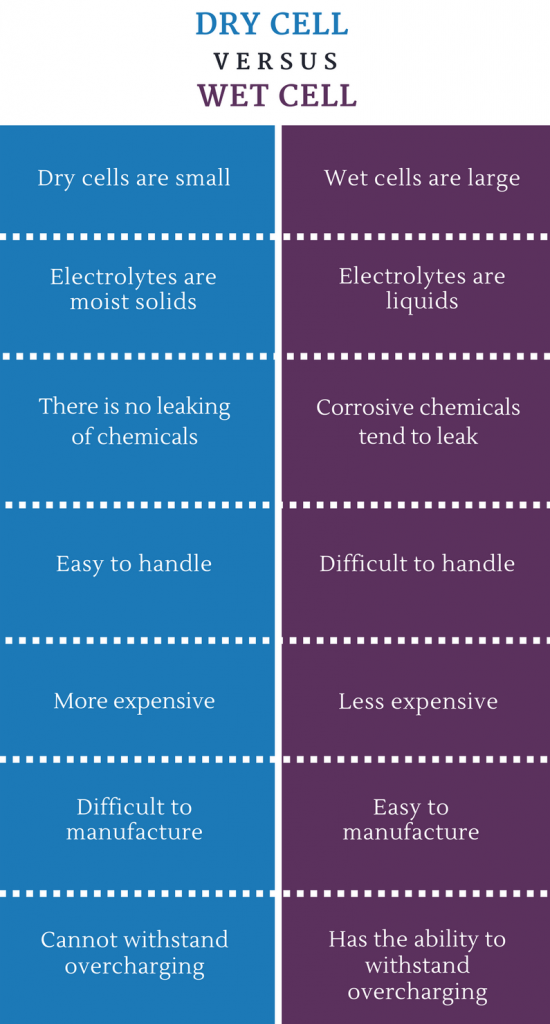
What is the difference between Dry cell and Wet Cell